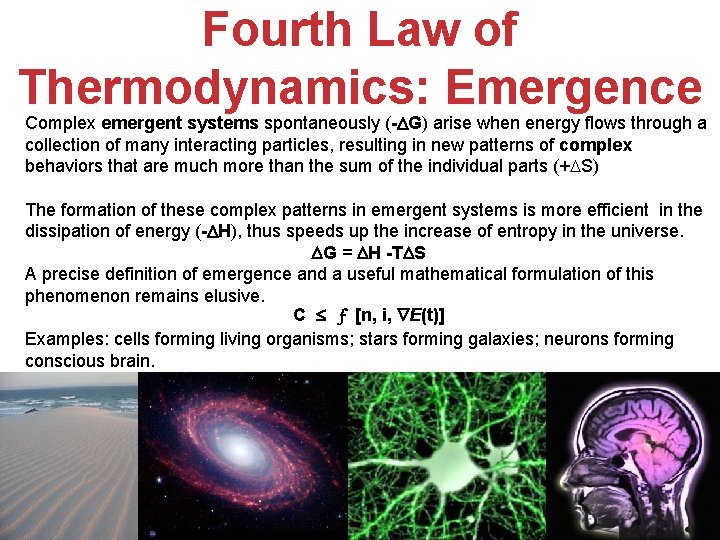
Fourth Law Of Thermodynamics
Fourth law of thermodynamics. First Law of Thermodynamics. In any process the total energy of the universe remains the same. The dissipative component of evolution is in a direction of steepest entropy ascent We propose to call the fourth law of thermodynamics a general modelling rule that captures a common essential feature of a wide range of models for the dynamical behaviour of systems far from equilibrium and therefore encompasses a large.
If playback doesnt begin shortly try restarting your device. We have located some comments on the internet some of which make some sense. The Fourth Law requires that identical systems under identical conditions behave identically.
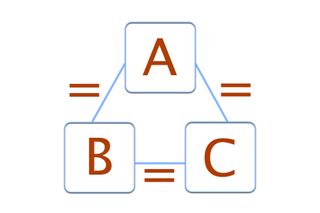
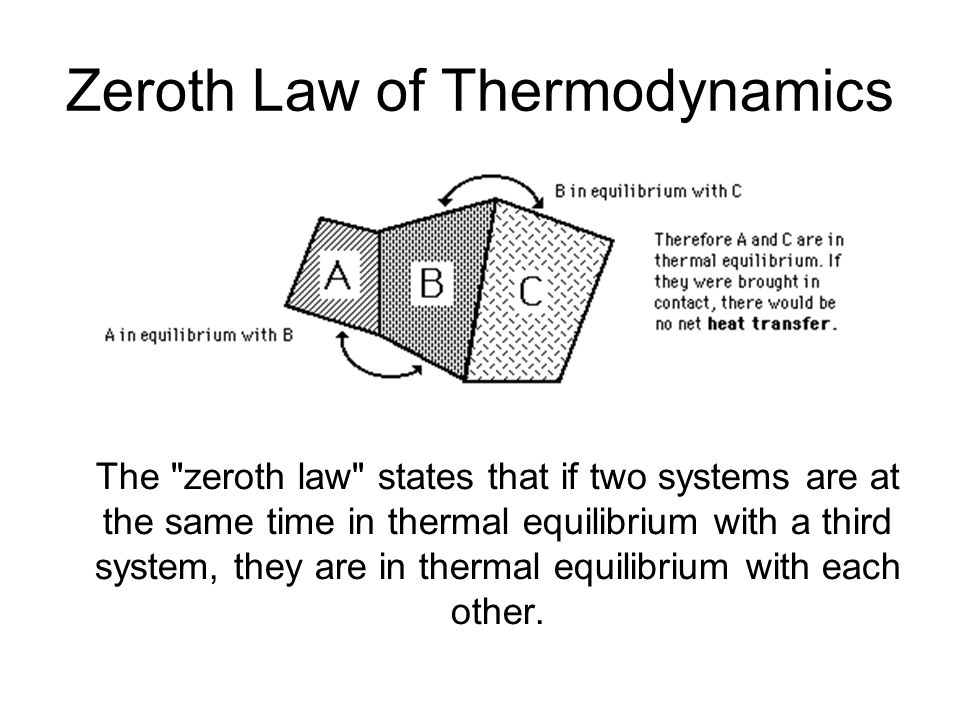
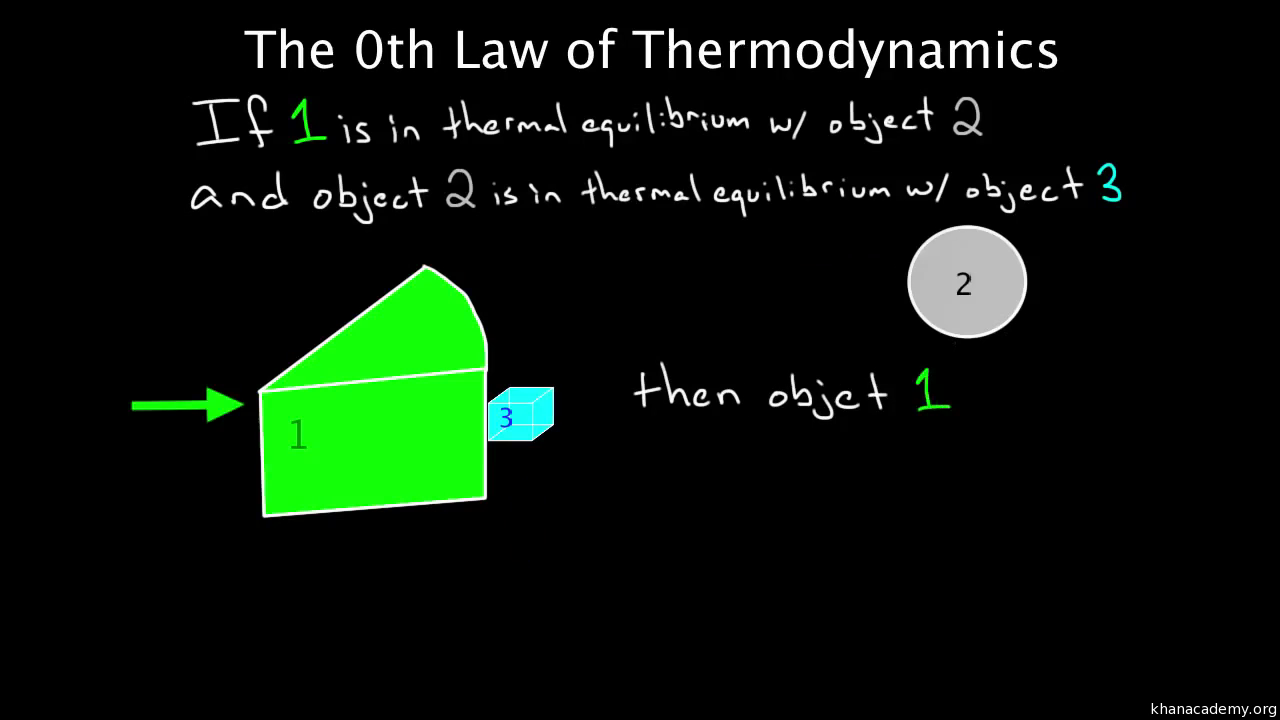
Therefore it has high entropy. Zeroth law of thermodynamics. Videos you watch may be added to.
New York and London June 1971 550. Systems increase entropy at the maximum rate available to them The law they say is universal and applies to every evolving system whether far. Fourth law of thermodynamics states that New Yorkers will expand into any living space and fill it with stuff.
This assertion of consistency applies to every evolving system whether far from equilibrium or near equilibrium. Morel andGeorge Fleck noting correctly that the classical laws of thermodynamicsare silentabout the means and pathways for achieving change propose in their words asimply stated yet powerful Fourth Law of Thermodynamics that. The fourth law of thermodynamics states that New Yorkers will expand into any living space and fill it with stuff.
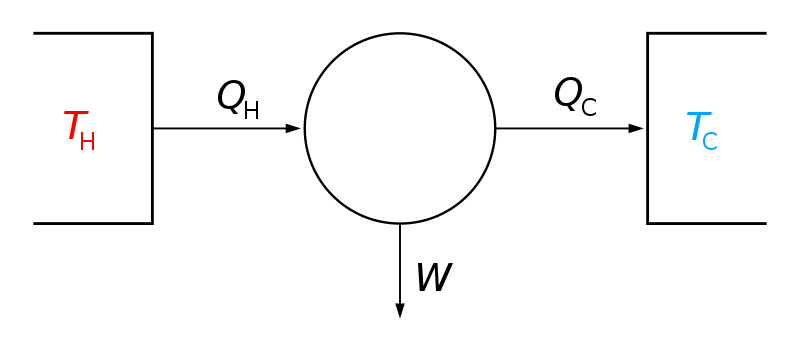
Second Law of Thermodynamics. Third Law of Thermodynamics Example The molecules within the steam move randomly. These laws are the fundamental building blocks on which all of thermodynamics rests.
Sys-tems increase entropy at the maximum rate available to them. Fourth law of thermodynamics.
These laws are the fundamental building blocks on which all of thermodynamics rests.
The Fourth Law of Thermodynamics. First law of thermodynamics Energy can neither be created nor destroyed. First Law of Thermodynamics. Zeroth law of thermodynamics. The fourth law seems to have resulted from attempts to relate the 0-3rd Laws to more complex non- thermodynamic systems. The fourth law does not yet seem to have made its way into the textbooks however I have been using older texts. Videos you watch may be added to. The 4th Law Explained. If playback doesnt begin shortly try restarting your device.
This assertion of consistency applies to every evolving system whether far from equilibrium or near equilibrium. The Fourth Law of Thermodynamics. The fourth law of thermodynamics states that New Yorkers will expand into any living space and fill it with stuff. In thermodynamics there are four laws which are called the Laws of Thermodynamics. Two systems are said to be in thermal equilibrium with respect to each other if they are linked by a wall permeable only to. Accordingly thermal equilibrium between systems is a transitive relation. Today in this article we will be going to discuss these four thermodynamics laws in a detailed manner.


































Post a Comment for "Fourth Law Of Thermodynamics"